iPhone Applications for the Science Classroom
 Tuesday, June 23, 2009 at 8:56AM
Tuesday, June 23, 2009 at 8:56AM I know what you are thinking. How can an iPhone be of any educational value in the science classroom? You are right. As of this moment it’s not quite ready to improve your students’ learning, but there are some tantalizing possibilities in the near future. What features does an iPhone have that can be used in the classroom?
1. An accelerometer. You have seen commercials showing the iPhone’s screen changing when the unit’s position is changed. How does it do that? It has a built in accelerometer that detects change in position in the x, y and z axis, as well as a change in speed. Speed and direction together is velocity, and a change in velocity is acceleration. What are some apps that take advantage of the accelerometer?
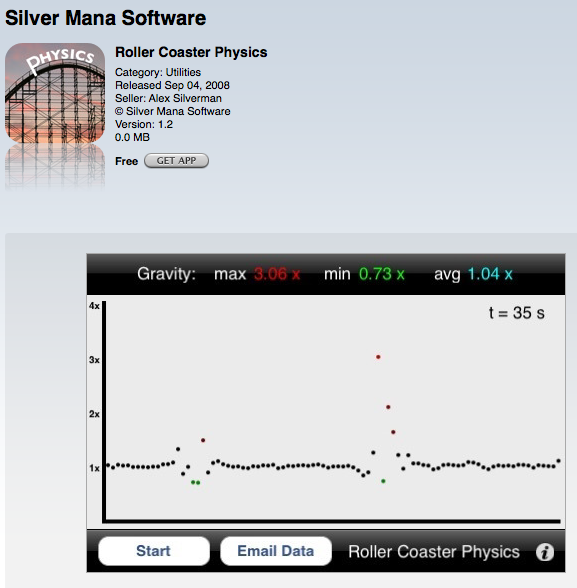
Roller Coaster Physics (Silver Mana Software) This app measures vertical g-forces. Press the start button and it will plot the vertical g-forces on the y axis and time on the x axis. This is ideally meant for a person to keep the iPhone in a pocket while riding a roller coaster. The best feature is emailing the data to yourself. The data is in comma separated value form and can easily be imported into Excel for analysis.
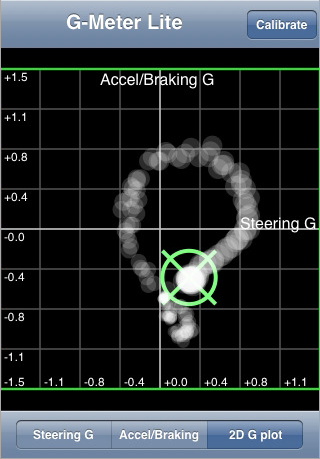
G-Meter Lite (Silverview Consulting Inc.) This app measures “steering” (left/right) g forces and accelerating/braking g forces. It plots the data points on a four quadrant graph. Unfortunately this app doesn’t save the data for use later analysis. It is merely a visual representation of real time forces, and thus it really isn’t useful for data analysis. G FORCE and G-Force are also apps that do this very same thing.
2. A microphone. The iPhone obviously has a microphone or else it couldn’t be used as a phone. What app can possibley be used in a science class that takes advantage of the microphone?
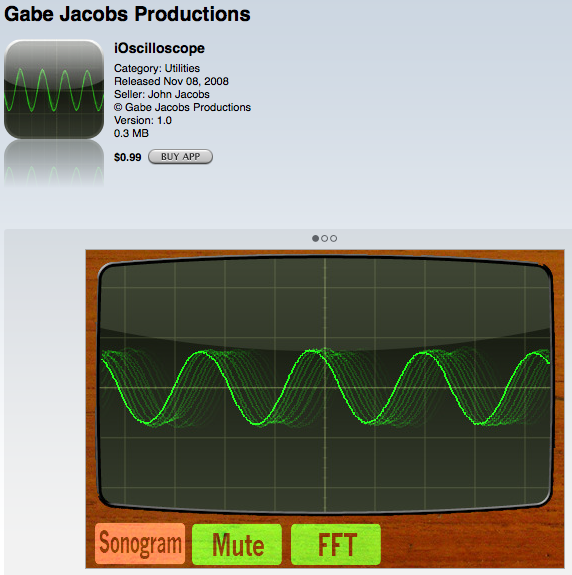
ioscilloscope (Gabe Jacobs Productions) This app shows the amplitude and wavelength of sound waves. Have a student bring in an instrument (or use prerecorded tones), like a trumpet or a sax and have the student play a single note at different volume levels to show the differences in amplitude. Then have the student play a series of higher pitched notes to show the different wavelengths (or frequencies).
3. A clock Don’t all cell phones have a clock? Yes, so the iPhone isn’t so special on this feature. Each year our science department buys 15+ stopwatches at about $6 each minimum. These are cheap stopwatches and their quality is also cheap. They don’t last more than 10-20 uses because either the battery goes dead or the buttons break. Since they don’t last more than a year they are considered consumables. This year I decided to end this cycle of spending. Most students now have cell phones and most of these cell phones have a stopwatch (timer) feature that measures to the nearest 0.01 seconds. Now when we need to measure time for learning activities I ask volunteers to use their own cell phones and the science department doesn’t have to spend any more money.
Summary It’s a little too soon to utilize a few of these iPhone apps to enhance learning in the science classroom. What is encouraging though is in probably 5 years most cell phones will be like the iPhone–a minicomputer that can collect all types of data. Today many physics and chemistry classes use expensive specialized probeware (like from Vernier and Pasco) to collect and upload data to computers. Students might bring their own data collection devices via their cell phones.
 applications
applications